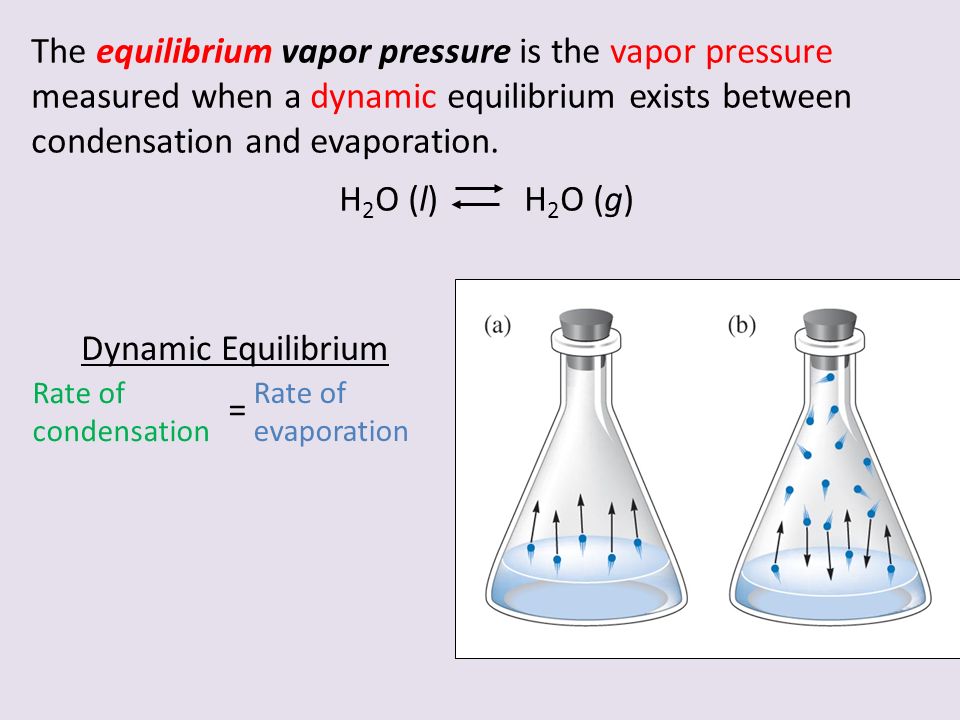
Why Does Equilibrium Vapor Pressure Increase With Temperature . For example, consider the vapor pressure of water between 0°c and 100 °c, shown in. the increase in vapor pressure with temperature is not linear, however. We would expect it to get larger as the. The natural logarithm (ln) changes the nonlinear. How does the vapor pressure change with temperature? vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. the equilibrium vapor pressure of a substance at a particular temperature is a characteristic of the material, like its molecular mass, melting point, and. the pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. vapor pressure and temperature.
from www.vrogue.co
the pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in. vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. the equilibrium vapor pressure of a substance at a particular temperature is a characteristic of the material, like its molecular mass, melting point, and. the increase in vapor pressure with temperature is not linear, however. The natural logarithm (ln) changes the nonlinear. How does the vapor pressure change with temperature? vapor pressure and temperature. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. We would expect it to get larger as the.
Physical Equilibrium Vapor Pressure And Boiling Point vrogue.co
Why Does Equilibrium Vapor Pressure Increase With Temperature vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. the pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. How does the vapor pressure change with temperature? vapor pressure and temperature. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in. vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. the increase in vapor pressure with temperature is not linear, however. We would expect it to get larger as the. The natural logarithm (ln) changes the nonlinear. the equilibrium vapor pressure of a substance at a particular temperature is a characteristic of the material, like its molecular mass, melting point, and.
From chempedia.info
H2SO4 vapor pressure over sulfuric acid Big Chemical Encyclopedia Why Does Equilibrium Vapor Pressure Increase With Temperature The natural logarithm (ln) changes the nonlinear. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in. vapor pressure and temperature. vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. We would expect it to get larger as. Why Does Equilibrium Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Liquids, solids, & intermolecular forces PowerPoint Presentation Why Does Equilibrium Vapor Pressure Increase With Temperature The natural logarithm (ln) changes the nonlinear. vapor pressure and temperature. the equilibrium vapor pressure of a substance at a particular temperature is a characteristic of the material, like its molecular mass, melting point, and. the pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is. Why Does Equilibrium Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Why Does Equilibrium Vapor Pressure Increase With Temperature We would expect it to get larger as the. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in. vapor pressure and temperature. How does the vapor pressure change with temperature? the increase in vapor pressure with temperature is not linear, however. the equilibrium vapor pressure of a substance at a particular. Why Does Equilibrium Vapor Pressure Increase With Temperature.
From chemistry.stackexchange.com
physical chemistry Why does increase in pressure favor formation of Why Does Equilibrium Vapor Pressure Increase With Temperature the increase in vapor pressure with temperature is not linear, however. vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. vapor pressure and temperature. How does the vapor pressure change with temperature? For example, consider the vapor pressure of water between 0°c. Why Does Equilibrium Vapor Pressure Increase With Temperature.
From www.youtube.com
The Effect of Pressure Changes on Equilibrium YouTube Why Does Equilibrium Vapor Pressure Increase With Temperature vapor pressure and temperature. the pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. the equilibrium vapor pressure of a substance at a particular temperature is a characteristic of the material, like its molecular mass, melting point, and. The natural logarithm. Why Does Equilibrium Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT The Haber Process PowerPoint Presentation, free download ID6194698 Why Does Equilibrium Vapor Pressure Increase With Temperature the equilibrium vapor pressure of a substance at a particular temperature is a characteristic of the material, like its molecular mass, melting point, and. The natural logarithm (ln) changes the nonlinear. How does the vapor pressure change with temperature? vapor pressure and temperature. vapor pressure is the pressure exerted by a vapor above its liquid (or solid). Why Does Equilibrium Vapor Pressure Increase With Temperature.
From chem.libretexts.org
11.5 Vapor Pressure Chemistry LibreTexts Why Does Equilibrium Vapor Pressure Increase With Temperature the equilibrium vapor pressure of a substance at a particular temperature is a characteristic of the material, like its molecular mass, melting point, and. The natural logarithm (ln) changes the nonlinear. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in. the pressure exerted by the gas in equilibrium with a solid or. Why Does Equilibrium Vapor Pressure Increase With Temperature.
From www.researchgate.net
Equilibrium vapor pressures as a function of temperature for 9 Why Does Equilibrium Vapor Pressure Increase With Temperature the increase in vapor pressure with temperature is not linear, however. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. the pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. For example, consider the vapor. Why Does Equilibrium Vapor Pressure Increase With Temperature.
From www.vrogue.co
Physical Equilibrium Vapor Pressure And Boiling Point vrogue.co Why Does Equilibrium Vapor Pressure Increase With Temperature the pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. vapor pressure and temperature. the increase in vapor pressure with temperature is not linear, however. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in. . Why Does Equilibrium Vapor Pressure Increase With Temperature.
From www.researchgate.net
Calculated equilibrium vapor pressures over SrTiO 3 at 1000 C as Why Does Equilibrium Vapor Pressure Increase With Temperature the increase in vapor pressure with temperature is not linear, however. vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. the equilibrium vapor pressure of a substance at a particular temperature is a characteristic of the material, like its molecular mass, melting. Why Does Equilibrium Vapor Pressure Increase With Temperature.
From www.showme.com
Liquid Vapor Equilibrium Science, Chemistry, Gases ShowMe Why Does Equilibrium Vapor Pressure Increase With Temperature We would expect it to get larger as the. vapor pressure and temperature. How does the vapor pressure change with temperature? the increase in vapor pressure with temperature is not linear, however. the pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor. Why Does Equilibrium Vapor Pressure Increase With Temperature.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo Why Does Equilibrium Vapor Pressure Increase With Temperature For example, consider the vapor pressure of water between 0°c and 100 °c, shown in. We would expect it to get larger as the. the equilibrium vapor pressure of a substance at a particular temperature is a characteristic of the material, like its molecular mass, melting point, and. the pressure exerted by the gas in equilibrium with a. Why Does Equilibrium Vapor Pressure Increase With Temperature.
From www.youtube.com
Effect of Temperature on Equilibrium Constants (Extension Material Why Does Equilibrium Vapor Pressure Increase With Temperature We would expect it to get larger as the. vapor pressure and temperature. the pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. The natural logarithm (ln) changes the nonlinear. generally a substance's vapor pressure increases as temperature increases and decreases. Why Does Equilibrium Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation ID1757765 Why Does Equilibrium Vapor Pressure Increase With Temperature vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. How does the vapor pressure change with temperature? For example, consider the vapor pressure of water between 0°c and. Why Does Equilibrium Vapor Pressure Increase With Temperature.
From learncheme.com
vaporliquidequilibriumdiagramfornonidealmixtures LearnChemE Why Does Equilibrium Vapor Pressure Increase With Temperature the pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. We would expect it to get larger as the. vapor pressure and temperature. vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at. Why Does Equilibrium Vapor Pressure Increase With Temperature.
From www.chegg.com
Solved 1. Why does equilibrium vapor pressure increase with Why Does Equilibrium Vapor Pressure Increase With Temperature the pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. vapor pressure and temperature. We would expect it to get larger as the. vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at. Why Does Equilibrium Vapor Pressure Increase With Temperature.
From circuitwiringtray.z13.web.core.windows.net
Diagram Of Vapor Pressure Why Does Equilibrium Vapor Pressure Increase With Temperature For example, consider the vapor pressure of water between 0°c and 100 °c, shown in. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. We would expect it. Why Does Equilibrium Vapor Pressure Increase With Temperature.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent Why Does Equilibrium Vapor Pressure Increase With Temperature We would expect it to get larger as the. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. The natural logarithm (ln) changes the nonlinear. the increase. Why Does Equilibrium Vapor Pressure Increase With Temperature.